CD34+ Cell Therapy Technology for Ischemic Repair
What is Regenerative Medicine?
Regenerative medicine is an interdisciplinary field that combines principles of biology and engineering to develop therapies for diseases characterized by cell depletion, lost tissue, or damaged organs. Ultimately, the goal of regenerative medicine is to improve the daily well-being of patients with debilitating chronic diseases by developing a new generation of therapies using natural growth and repair mechanisms, such as stem cells. Cell therapy, which uses specific types of stem cells to repair damaged tissues and treat disease, is one approach of the regenerative medicine.
What is cell therapy?
The human body contains over 200 different specialized cell types, such as muscle, bone or brain cells. These cells carry out specific functions within the body, necessary for the health of an organism. Injury, disease, or ageing can lead to the loss of specialized cells from the body. In many cases, such loss is irreversible, meaning that the diseased or lost cells can no longer be replenished by healthy ones.
Cell therapy aims to introduce new, healthy cells into a patient’s body to grow, replace, or repair damaged tissue. Cell therapies may be autologous, meaning that the patient receives cells from their own body, or they may be allogenic, meaning the patient receives cells from a donor. When the donor and the recipient are not the same individual, the procedures are referred to as “allogeneic” cell therapy.
Various cell therapies are in clinical development for an array of human diseases, including autoimmune, oncologic, cardiovascular, neurologic, and orthopedic diseases. The FDA has been investing significant resources into policies and organizational changes to advance the cell and gene therapy fields in recent years through what the agency has labeled its “comprehensive regenerative medicine policy framework,” as first announced in late 2017 and incrementally developed since that time through various agency actions.
What are CD34+ stem cells?
The CD34+ cell was discovered as a result of the deliberate search for a stem cell capable of stimulating the development and/or repair of blood vessels. All tissues in the human body maintain their function by replacing cells over time. In addition to the maintenance function, the body must also be capable of building new blood vessels after injury. CD34+ cells are stem cells that can stimulate new blood vessel formation. No other native cell discovered to date has demonstrated the same capability as CD34+ cells to promote angiogenesis.
CD34+ Cell Therapy: Technology Overview
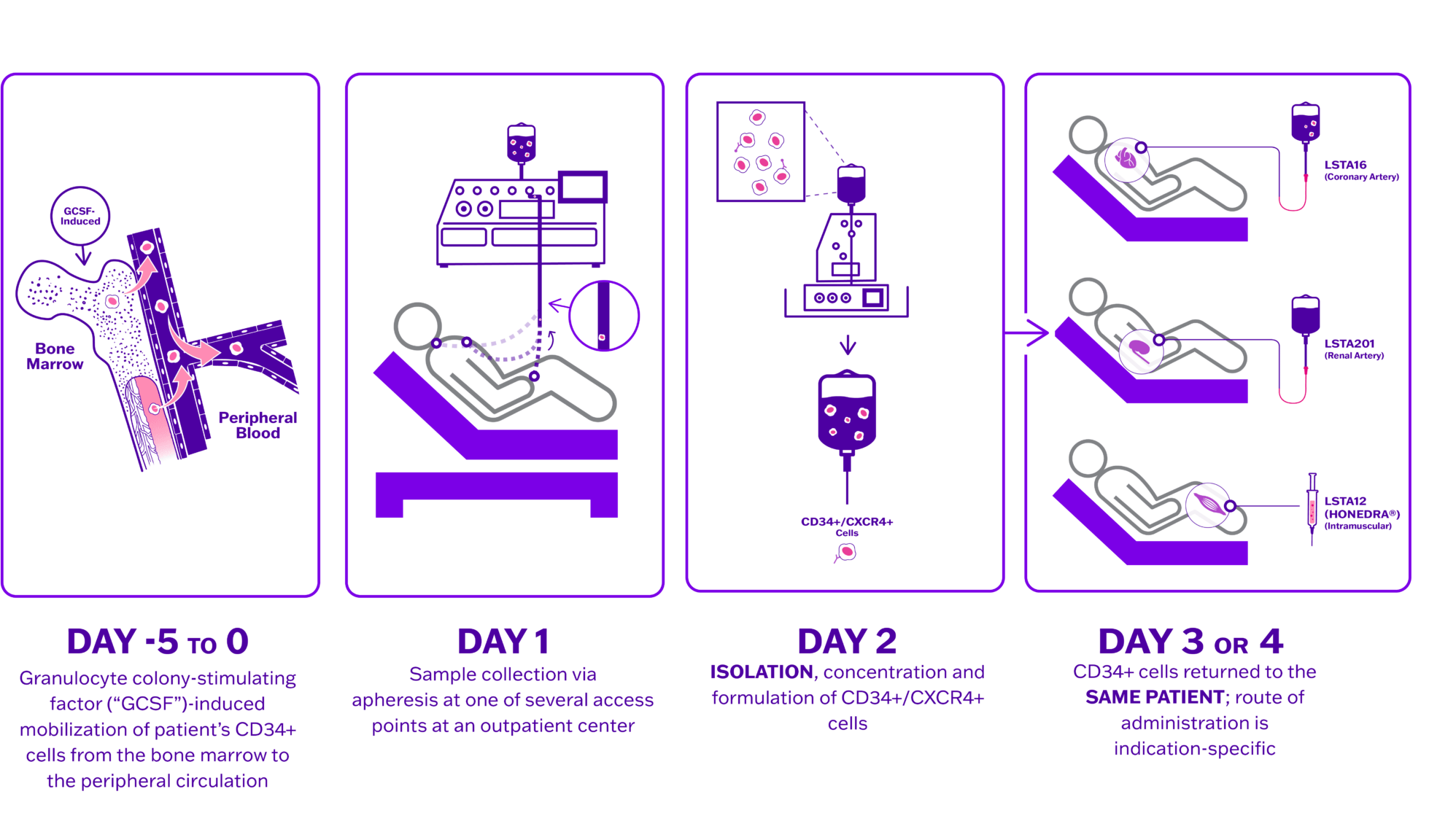
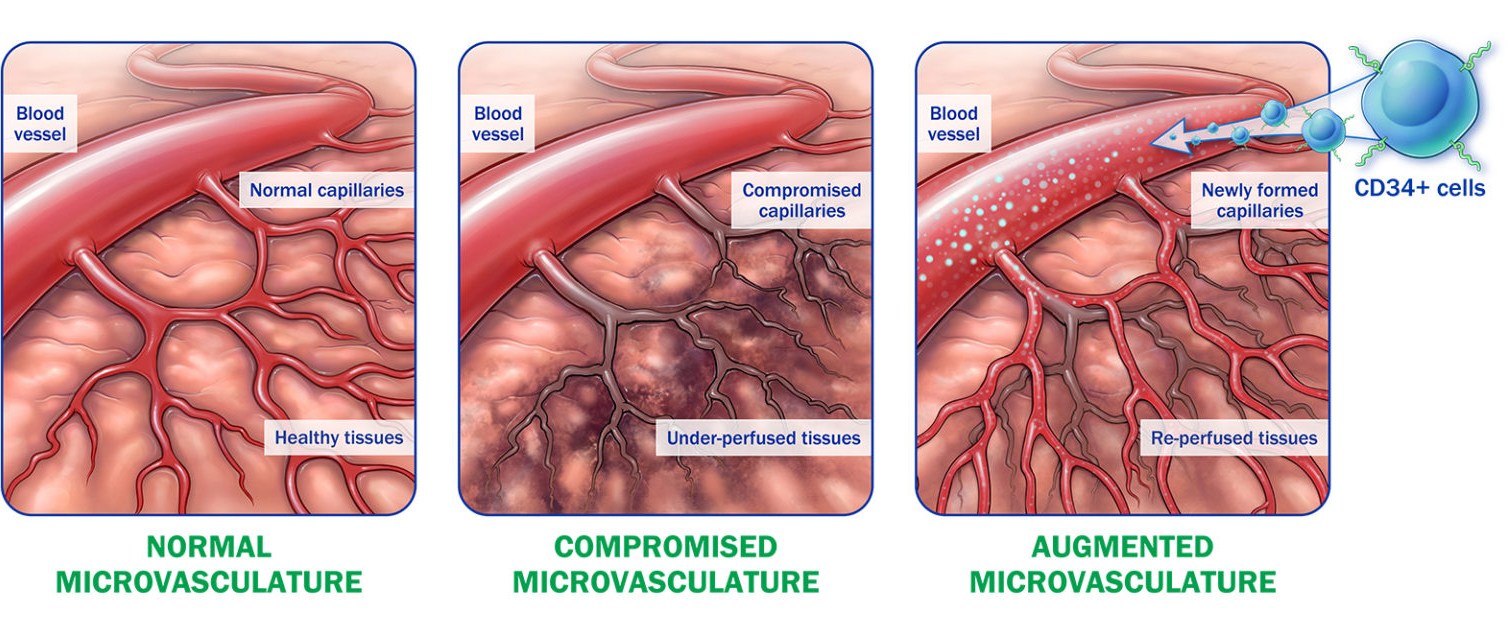
Lisata’s proprietary autologous CD34+ cell therapy platform has led to the development of therapeutic product candidates designed to address diseases and conditions caused by ischemia. Ischemia occurs when the supply of oxygenated blood to healthy tissue is restricted. Naturally occurring CD34+ cells that reside in the bone marrow can stimulate the development of new microvasculature throughout the body. The Company’s proprietary process involves mobilization of each patient’s CD34+ cells from the bone marrow followed by cell collection via apheresis, manufacturing, and delivery of the treatment to the patient. Through the administration of autologous CD34+ cells, the Company seeks to promote the development and formation of new microvasculature and thereby increase blood flow to the impacted area. Lisata believes that a number of conditions caused by underlying ischemic injury can be improved through its CD34+ cell therapy, including but not limited to, critical limb ischemia (CLI), coronary microvascular dysfunction (CMD), diabetic kidney disease (DKD) and no-option refractory disabling angina (NORDA).
CD34+ cell therapy is extensively studied and clinically validated
CD34+ cell therapy has been shown to consistently successfully treat a range of cardioCD34+ cell therapy has been shown to consistently successfully treat a range of cardiovascular conditions associated with ischemia. Randomized, double blinded, placebo controlled clinical trials involving more than 700 patients offer compelling patient data about the safety, therapeutic effect, and benefits from treating people with autologous CD34+ cells.
- Preclinical studies document improved microcirculation in myocardial infarction and DKD
- Phase 2 clinical studies in CMD and CLI consistently show benefits in safety and function
- Reduced amputation in critical limb ischemia
- Improved function in claudication
- Reduced angina and improved exercise tolerance testing in refractory angina
- Improved mortality and left ventricular ejection fraction in dilated cardiomyopathy
CD34+ cell therapy has a well characterized mechanism of action
- Naturally occurring endothelial progenitor cells that re-establish blood flow to under-perfused tissues1,2
- Possess pre-programmed pro-angiogenic repair properties3,4
The CD34+ cell therapy process is rapid, economical, and easily scalable compared to other autologous cell therapy processes
- Drug induced mobilization eliminates need for surgical bone marrow aspiration
- No genetic manipulation or ex vivo expansion of cells
- Four days or less from donation to treatment